why do all bonds form
The investor agrees to give the corporation a certain amount of money for a. The formation of bonds is explained in terms of changes in chemical potential energy as the distance between atoms changes.
 |
| Why Do Chemical Bonds Form Ppt Download |
Molecules are composed of atoms that have bonded and released the extra energy with them as bond formation energyThis bond can either be ionic or covalent.

. When this force of attraction brings atoms together to form substances containing two or more atoms the bond is called a chemical bond. Hope it helps. When you buy a bond you are lending to the issuer which may be a government municipality or corporation. What is the rule for figuring out if it is ionic or covalent.
Forces of attraction and repulsion are referred to. When the outer electron orbital of an atom lacks an electron it seeks to bind itself to another atom such that its outer electron orbital will be filled. The metal atom forms a cation and the nonmetal atom forms an anion. Covalent bonds form between two metals.
The attraction between ions with the same charge. The more electronegative atoms transfer one or more electrons to the less electronegative atom. Issuing bonds is one way for companies to raise money. A bond that forms when 2 ions of opposite charge.
Other sets by this creator. An atom always prefers its most stable configuration. This type of chemical bond is called a covalent bond. During the formation of the ionic bond one of the reacting atoms will donate electrons and form positive ion.
Physical Sciences Grade 11. The more crowded a given space is with atoms the more likely it is that chemical bonding will occur. These reasons are noted below. Learners are reminded why noble gases do not form bonds.
This can either be a complete charge as is the case with ionic bonds or a partial charge as is the case with covalent bonding and most forms of. The more electronegative atoms transfer one or more electrons to the less electronegative atom. A covalent bond is a bond in which two atoms share one or more pairs of electrons. Borrowers issue bonds to raise money from investors willing to lend them money for a certain amount of time.
There are many types of chemical bonds but the three major or. The less electronegative atoms transfers one or more electrons to the more electronegative atom. Bonds are formed because every atom wants to stabilise itself. As a result the riskier the issuer the higher the interest rate will be.
Covalent bonds form between two metals. Covalent bonds form between a metal and a non-metal. Atoms form bonds with other atoms because of the electrostatic attraction between positively-charged protons and negatively-charged electrons. A bond that forms when 2 atoms take valence electrons.
A corporation has a choice of raising money by selling shares or by issuing bonds. Atoms form chemical bonds to make their outer electron shells more stable. Ionic bonds form between metal atoms and nonmetal atoms. Atoms have to be close together to form a bond.
But sometimes molecules form coordinate bondsThis results in a complex a compound formed due to coordinate bonding between two moleculesFor ex ammonia-boron triflouride complex. Ionic bonds form between two metals. They can do so by lowering their energy and bond formation is a type of exothermic process. There are specific reasons why the issuance of bonds is a better choice than issuing shares.
An ionic bond where one atom essentially donates an electron to another forms when one atom becomes stable by losing its outer electrons and the other atoms become stable usually by filling its valence shell by. The loss or gain of valence electrons allows ions to obey the octet rule and become more stable. Why they are formed. A bond is a debt security similar to an IOU.
A bond that forms when 2 atoms give valence electrons. A bond that forms when 2 atoms share valence electrons. Ionic bonds are formed through the exchange of valence electrons between atoms typically a metal and a nonmetal. Ionic bonds form between a metal and a non-metal.
A bond functions as a loan between an investor and a corporation. Hence whenever a bond is formed energy is released and lowers the energy of atom hence making it more stable. Atoms have to be charged to form a bond. Ionic bonds form between metal atoms and nonmetal atoms.
The less electronegative atoms transfers one or more electrons to the more electronegative atom. The type of chemical bond maximizes the stability of the atoms that form it. In return the issuer promises to pay you a specified rate of interest during the life of the bond and to repay the principal also. The single electrons from each of the two hydrogen atoms.
The metal atom forms a cation and the nonmetal atom forms an anion. Thus it forms chemical bonds with other atoms and rearranges until it is in its most stable. They usually involve metals and nonmetals as elements active in ionic bonds are mostly from opposite ends of the periodic table with an electronegativity difference exceeding 167. Why companies issue bonds.
Why do Ionic Bonds Form. Since bonds are debts if the issuer fails to pay back their debt the bond can default. The issuance of bonds essentially creates a loan between a group of investors and the corporation. Ionic bonds are formed when one of the two atoms that are reacting has excess electrons and transfer the electrons to the atom that is deficient in electrons.
In fact they have to collide bump together. The point at which the potential energy reached its minimum represents the ideal distance between hydrogen atoms for a stable chemical bond to occur. Chemical bonds occur to stabilize atoms. Ionic compounds are typically neutral.
An electrostatic attraction between ions with opposite charges cations and anions causes ionic bonds.
 |
| Single And Multiple Covalent Bonds Article Khan Academy |
 |
| Coordinate Covalent Bond Definition And Examples Covalent Bonding Chemical Bond Bond |
 |
| As Chemistry Revision Bonding Why Do Bonds Form N Bonding Holds Particles Together We Need To Input Energy To Break Them Bond Enthalpy N Substances Ppt Download |
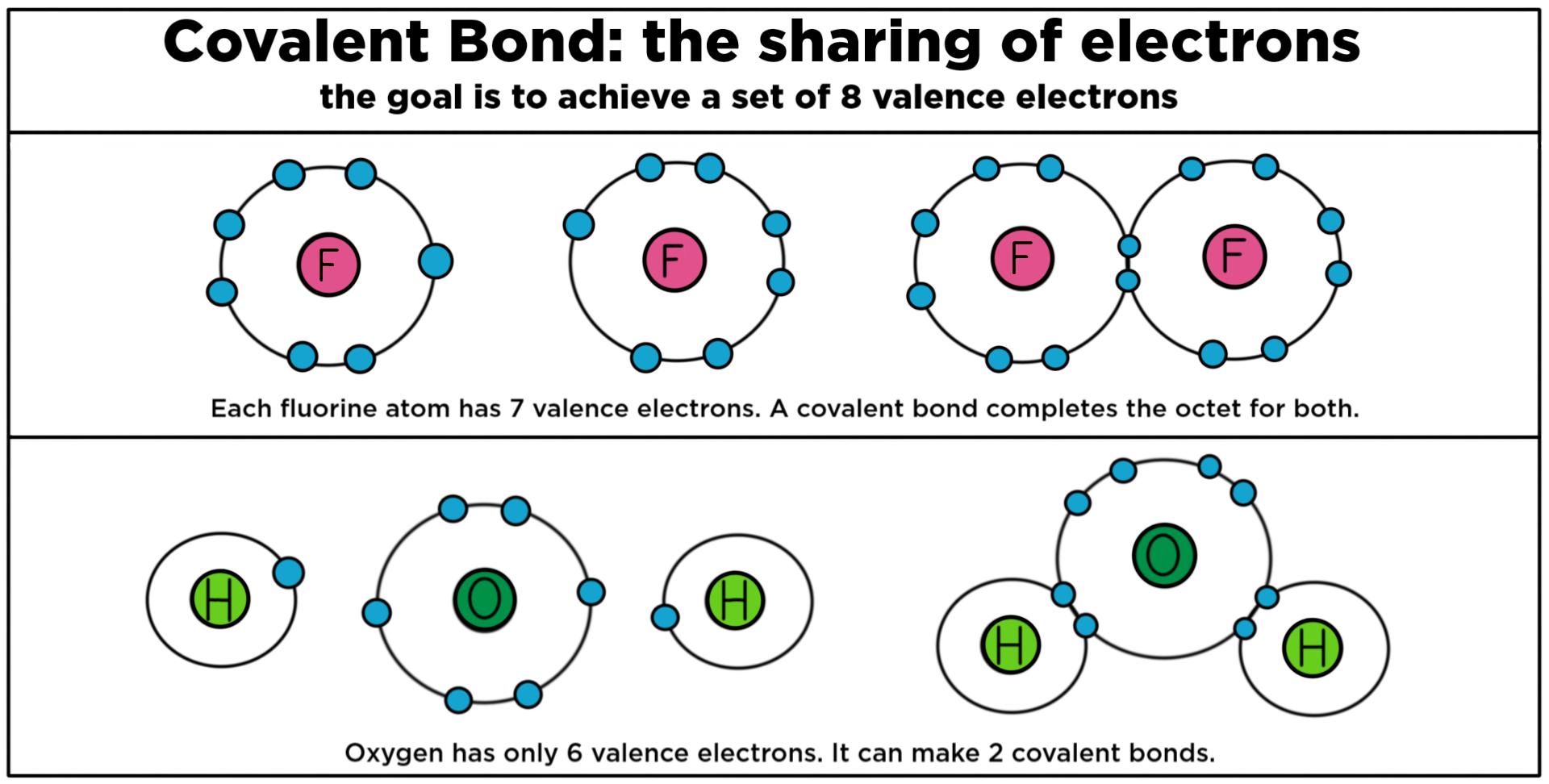 |
| Covalent Bonding Biology Definition Role Expii |
 |
| Why Do Atoms Form Chemical Bonds Socratic |
Posting Komentar untuk "why do all bonds form"